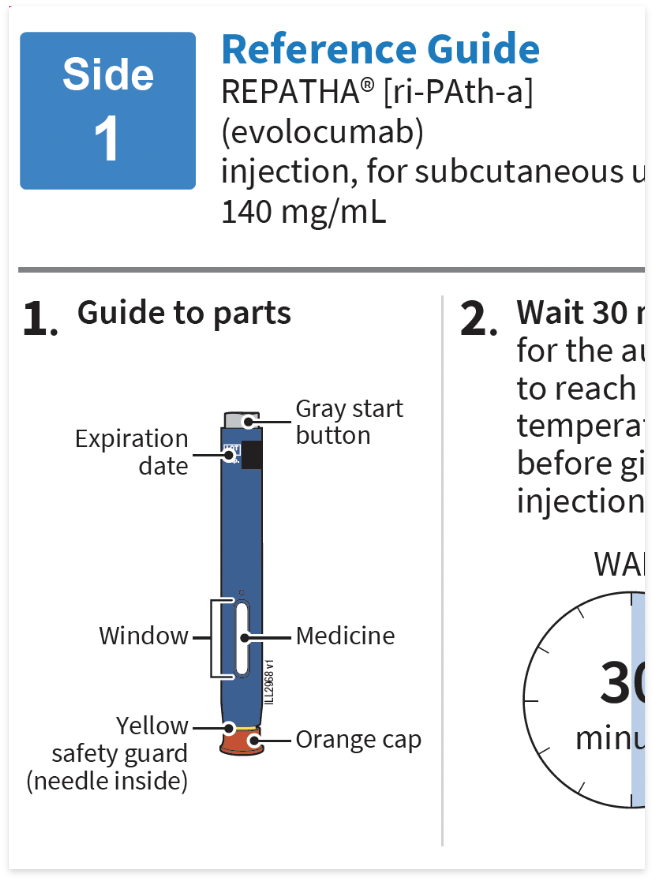
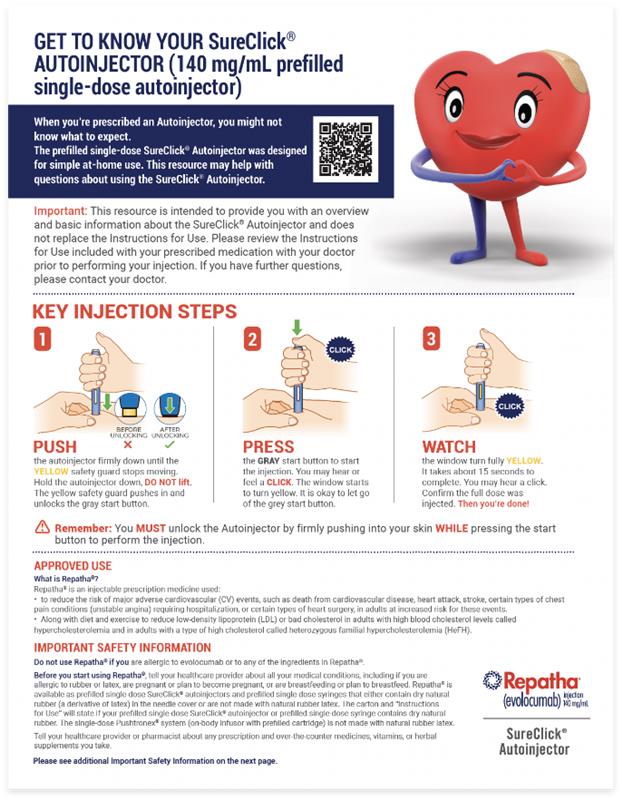
Approved Use
Repatha® is an injectable prescription medicine used:
- to reduce the risk of major adverse cardiovascular (CV) events, READ MOREsuch as death from cardiovascular disease, heart attack, stroke, certain types of chest pain conditions (unstable angina) requiring hospitalization, or certain types of heart surgery, in adults at increased risk for these events.
- to reduce the risk of major adverse cardiovascular (CV) events, such as death from cardiovascular disease, heart attack, stroke, certain types of chest pain conditions (unstable angina) requiring hospitalization, or certain types of heart surgery, in adults at increased risk for these events.
- Along with diet and exercise to reduce low-density lipoprotein (LDL) or bad cholesterol in adults with high blood cholesterol levels called hypercholesterolemia and in adults with a type of high cholesterol called heterozygous familial hypercholesterolemia (HeFH).
Repatha® is an injectable prescription medicine used:
- to reduce the risk of major adverse READ MORE cardiovascular (CV) events, such as death from cardiovascular disease, heart attack, stroke, certain types of chest pain conditions (unstable angina) requiring hospitalization, or certain types of heart surgery, in adults at increased risk for these events.
- Along with diet and exercise to reduce low-density lipoprotein (LDL) or bad cholesterol in adults with high blood cholesterol levels called hypercholesterolemia and in adults with a type of high cholesterol called heterozygous familial hypercholesterolemia (HeFH).
For adults at increased risk of a major adverse cardiovascular event